Comparing sets of trees from different analyses
Martin R. Smith
Source:vignettes/compare-treesets.Rmd
compare-treesets.RmdA common application of tree space analysis is to compare the outputs of different analyses – for instance, trees obtained from different gene sequences, or results obtained using different models or methods (e.g. Bayesian, maximum likelihood, or parsimony).
Shiny app
This can be accomplished quickly using the MapTrees()
graphical user interface:
Load trees from file: Select first tree file
Select an appropriate sample size
Select Replace existing
Load each additional set of trees from file using Add batch to existing
- On the Display tab, select Point symbols: One per batch, or Colour points by: Batch
Scripting at the R command line
More control over the mapping can be obtained at the command line:
# Load trees
library("TreeTools", quietly = TRUE)
batch1 <- as.phylo(1:60, 8) # Generate 60 similar trees
batch2 <- as.phylo(seq(200, 800, length.out = 30), 8) # A separate batch of 30 trees
styles <- c(1, 2) # Select plotting colours / symbols
treeStyle <- rep(styles, c(length(batch1), length(batch2)))
# Calculate distances
library("TreeDist")
distances <- ClusteringInfoDistance(c(batch1, batch2))
# Construct over-simple 2D PCoA mapping
mapping <- cmdscale(distances, k = 2)
# Plot mapping
par(mar = rep(0, 4))
plot(mapping,
asp = 1, # Preserve aspect ratio - do not distort distances
ann = FALSE, axes = FALSE, # Don't label axes: dimensions are meaningless
col = treeStyle, # Colour
pch = treeStyle # Plotting symbol
)
legend("left", c("Batch 1", "Batch 2"), col = styles, pch = styles)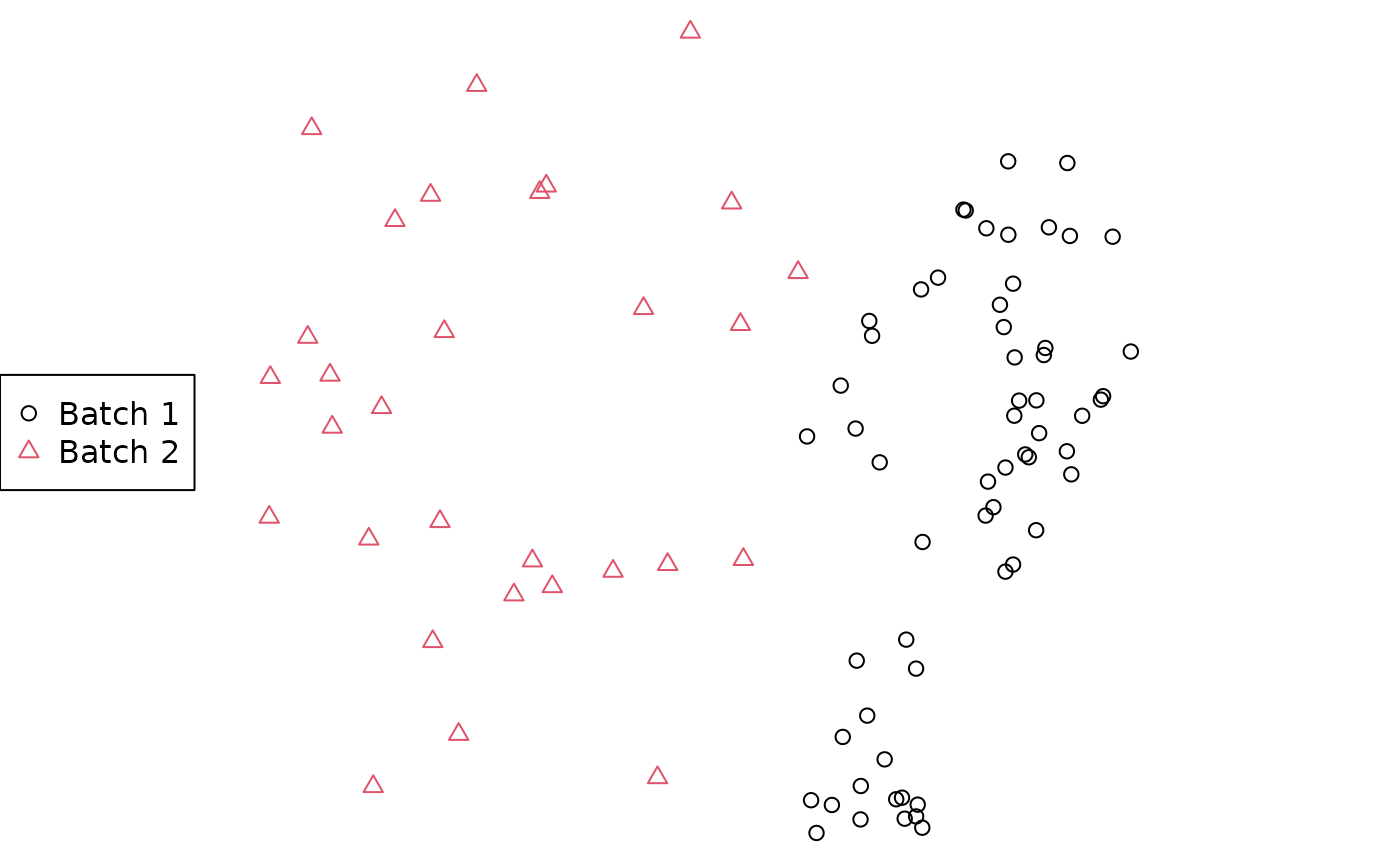
For more robust analyses than the (potentially misleading!) 2D plot above, consult the companion vignette. Note also that mapped areas and their regions of overlap may not correspond to reality; see the warnings and recommendations in Smith (2022a).
Comparing trees’ dispersal / hypervolume
Interpreting and comparing the areas of tree space from a projection can be misleading – the expanded apparent area of Greenland under the Mercator projection being a familiar example.

As such, it is always best to work with original distances when interpreting whether sets of trees occupy larger or smaller regions of tree space.
Distances from median
One approach is to plot distances from a median tree:
# Calculate median trees
median1 <- median(batch1)
median2 <- median(batch2)
# Compute distance from each tree to the median of its batch
dist1 <- ClusteringInfoDist(batch1, median1)
dist2 <- ClusteringInfoDist(batch2, median2)
# Set resolution of histogram
nBreaks <- 10
breaks <- seq(0, max(dist1, dist2), length.out = nBreaks)
# Plot first distance set
hist(dist1, col = "#00000022", breaks = breaks,
main = "Distance from median of batch",
xlab = "Clustering information distance",
ylim = c(0, 25) # Omit this line to infer Y axis limit from first batch.
)
# Add second distance set
hist(dist2, col = "#ff000022", breaks = breaks, add = TRUE)
# Add legend
legend("topleft", c("Batch 1", "Batch 2"),
fill = c("#00000022", "#ff000022"))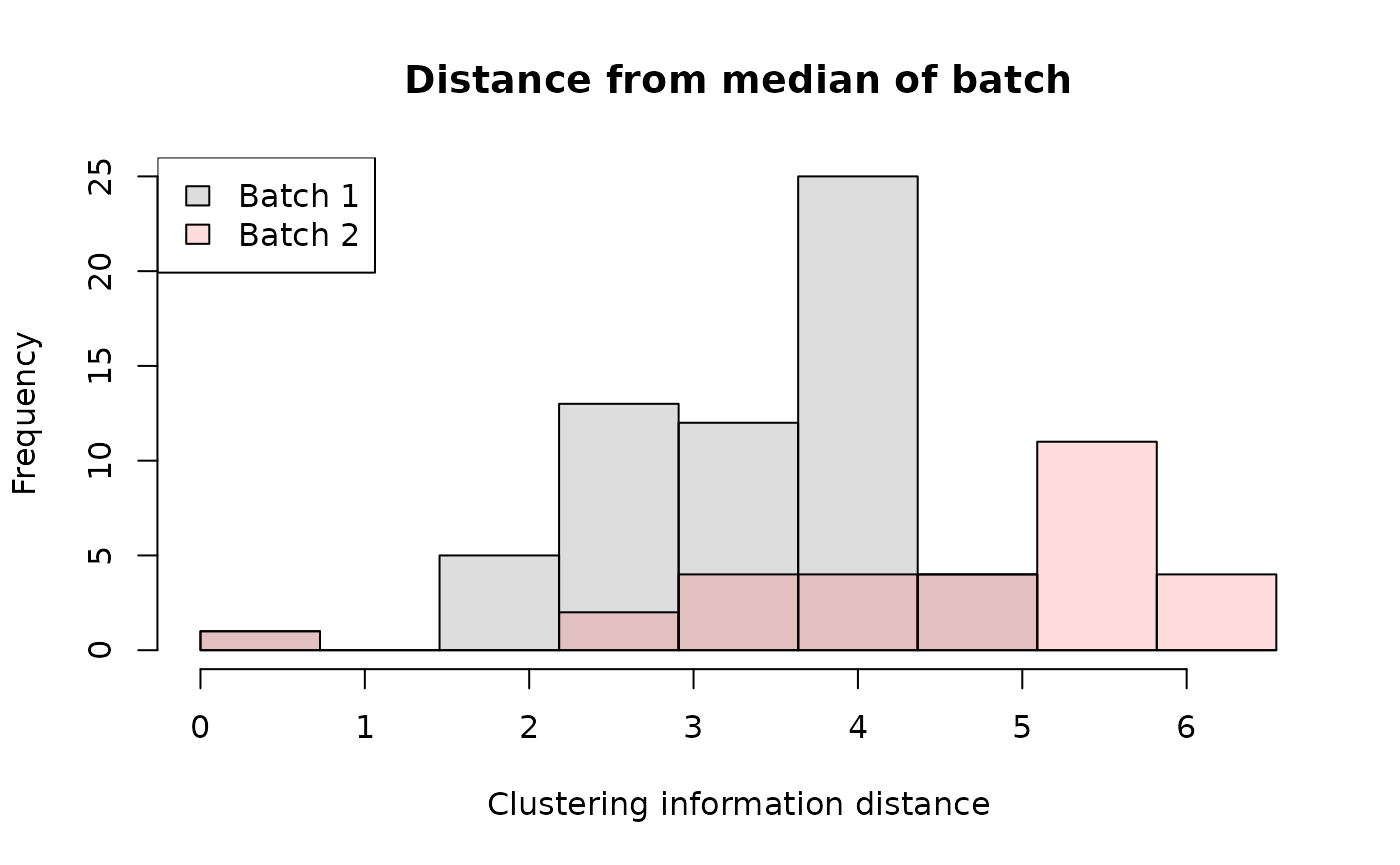
In the plotted example, distances to the median tree are greater for batch 2 than batch 1, indicating a more dispersed set of trees that occupies a greater hypervolume. Note that the increased frequency at higher distances is expected: the outer shell of a sphere contains more volume than a layer of equivalent thickness closer to the centre, and this phenomenon becomes more pronounced as the dimensionality of tree space increases.
Consensus resolution
A complementary approach is to identify the resolution of the consensus of each batch of trees. This approach shares many of the problems with the Robinson–Foulds distance: in particular, resolution can be decimated by a single “rogue” taxon whose position is poorly defined (Smith, 2022b). Detecting and removing rogue taxa can provide a more meaningful point of comparison.
# Create tree set with a rogue taxon
batch3 <- AddTipEverywhere(as.phylo(7, 7), "t8")
# Set up plotting area
par(mfrow = c(2, 2), mar = rep(0.4, 4))
# Plot naive strict consensus
plot(consensus(batch1, p = 1))
plot(consensus(batch3, p = 1))
if (requireNamespace("Rogue", quietly = TRUE)) {
cons1 <- ConsensusWithout(batch1, p = 1,
Rogue::QuickRogue(batch1, p = 1)[-1, "taxon"])
cons3 <- ConsensusWithout(batch3, p = 1,
Rogue::QuickRogue(batch3, p = 1)[-1, "taxon"])
# The information content of each tree gives a measure of its resolution,
# accounting for omitted rogue leaves
SplitwiseInfo(cons1) # 8.5 bits
SplitwiseInfo(cons3) # 15.1 bits: higher resolution indicates that these
# trees are more similar, notwithstanding rogue taxa.
# Plot the trees
plot(cons1)
plot(cons3)
} else {
message("The package 'Rogue' is required to run this example.")
}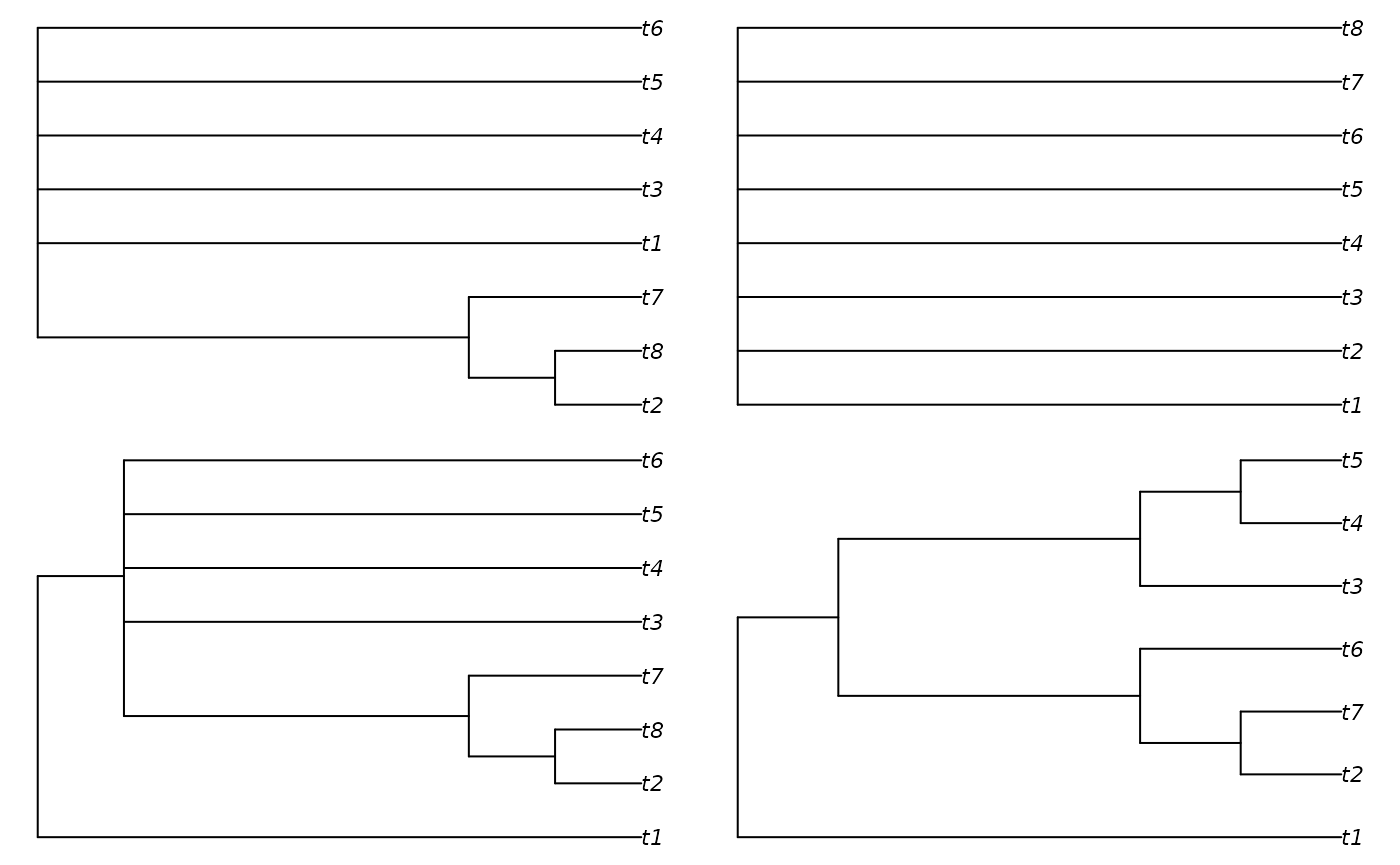
Whereas a direct interpretation of this analysis is not straightforward, it can provide a complementary way of understanding the distribution of trees across tree space.